Dr. Tien-Yau Luh
National Taiwan University,Taiwan
Title: Mimicking DNA Chemistry and Beyond-From Ladderphane to Stromaphane
Biography
Biography: Dr. Tien-Yau Luh
Abstract
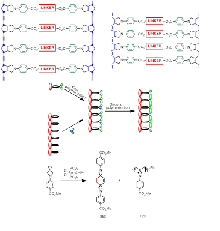
A ladderphane is a ladder-like polymer constituted of multiple layers of rigid linkers covalently linked to two or more polymeric backbones. The linkers can be planar aromatic, antiaromatic, macrocyclic metal complexes, or three-dimensional organic or organometallic moieties. Structurally, a DNA molecule is a special kind of ladderphane, where the cofacially aligned base-pair pendants are connected complementarily through hydrogen bonding.
Two norbornene or cyclobutene moieties fused with N-arylpyrrolidine are employed to connect covalently with rigid linkers. Ring opening metathesis polymerizations (ROMP) of these monomers using the ruthenium or molybdenum catalyst give the corresponding symmetrical double-stranded ladderphanes. Depending on the catalyst, double bonds in these ladderphanes can be either E or Z selectively. The presence of N-arylpyrrolidene moiety is crucial to control the isotactic stereoselectivity. The linkers in these ladderphanes are aligned coherently along the longitudinal axis of the polymer. Strong interactions between them may take place as evidenced by fluorescence quenching, excimer formation, Soret band splitting or electron hopping. Chiral helical ladderphanes are synthesized by incorporating chiral linkers.
These ladderphanes can easily aggregate to form a two-dimensional highly ordered array on graphite surface up to submicron area as revealed by scanning transmission microscopy (STM). Such assembly furnishes an entry to orient planar arene moieties cofacially, while each linear array of such arenes is insulated from the adjacent arrays by the polymeric backbones.
Sequential polymerization of a monomer having two different polymerizable groups or replication protocol offers useful entries for unsymmetical ladderphanes. This route furnishes template synthesis of daughter polymers with well-controlled chain lengths and polydispersity.
When cyclopropene having spirally connected N-ary azetidine is used for ROMP, substituted alt-methylene-vinylene with all double bonds in trans configuration is obtained. The stereospecificity can be considered as mimicking proofreading and repair in DNA synthesis. Two-dimensional polymers obtained from ROMP of biscyclopropene will be